About Minerals & Crystals
A “mineral” is an inorganic substance that is composed of one or more chemical elements. By definition a mineral must be:
Naturally occurring - A mineral can not be synthetic or man made.
A solid - Liquid and gases can not be minerals.
Contain an ordered elemental structure - The atoms that make it up are arranged in an orderly fashion.
Have a definite chemical composition - In various occurrences of the mineral the chemical composition is nearly identical.
The chemical composition of a mineral is known as its elemental composition. Most minerals occur as compounds (a combination of several different elements). However, some minerals occur as chemical elements by themselves. These are known as native minerals.
Crystals are a solid substance that possess an organized crystalline structure that’s formed from atoms. The internal arrangement of atoms often result in external plane faces like those seen on a quartz crystal, however this is not a requirement to be classified as a crystal. Crystals can be classified differently than minerals due to the fact that some materials can be considered organic crystals. Since minerals are purely inorganic, an organic crystal cannot be a mineral. For example, proteins and sugars are solids that can form crystals, however because they’re organic substances, they can’t be categorized as minerals.
Impurities within solutions that form crystals can result in color changes as well as significant crystal structure alterations. Too many impurities within a crystal lattice during crystallization can produce crystals with small to large inclusions. These inclusions can alter the shape of the crystal as well.
Most minerals will occur naturally as crystals, however not all crystals are minerals since organic crystals are not minerals at all. A mineral with the same chemical formula can form more than one type of crystal. For example, there are three polymorphs (same chemical formula, different crystalline structures) of calcium carbonate that are known as calcite, aragonite and vaterite. Calcite crystals occur in the trigonal system, aragonite crystals belong to the orthorhombic system and vaterite crystals form in the hexagonal system. These crystalline structures can vary during formation as a result of several factors which include impurities inhibiting growth patterns, the temperature of the environment during formation, the saturation of minerals within the solution, the geometry of the covalent bonds and changes in motion of the solution.
Minerals have a naturally formed, organized atomic structure with a specific chemical composition. Crystals for the most part will share these features, however the atoms are arranged in a repeating pattern that results in a crystal lattice, often presenting itself with crystal faces.
Many times you will hear people call minerals or crystals, rocks, however a “rock” is defined as a bound aggregate of minerals, mineraloids or fragments of other rocks. The word “bound” meaning the aggregation of minerals must be in a sense cemented together. For example, sand is not considered a rock even though in most cases sand grains are aggregated together. Sandstone for instance has become a rock because the sand grains have been cemented together by finer grained minerals and/or organic material, forming a relatively solid mass.
The three major classifications of rocks are igneous, metamorphic and sedimentary.
Igneous Rock - Rock that formed by the cooling and crystallization of magma within or above the lithosphere (earth’s crust). Crystals form within the magma as it begins to solidify, of which the speed of cooling can dictate the size of the crystals that form.

A close up view of porphyritic granite, a type of igneous rock. Porphyritic granite occurs when the temperature of the magma cooling changes quickly. In this instance, large crystals were allowed to form with slow cooling, only to be interrupted by a sudden decreasing temperature change that sped up the crystallization process, resulting in smaller crystals.
Sedimentary Rock - Rock that formed as a result of eroded materials from prior formed rocks being deposited along ocean floors, river beds, lake beds, etc., along with deposition of minerals from the water. Over years these depositions are compacted by natural forces, later to solidify into a solid mass (rock).
Metamorphic Rock - Rock that occurred when existing sedimentary or igneous rock was exposed to pressures and in some cases temperature changes that altered their original mineralogy.
Crystals can form from a variety of different processes, including:
Evaporite Deposits - These are mineral formations that occur as a result of processes at the earth’s surface. Crystals form from solutions containing minerals that become concentrated by dehydration/evaporation of an aqueous solution. As the fluid is slowly removed by evaporation, the concentrated minerals gather and precipitate from the water in a structured pattern that develops into a crystal. An example of precipitation deposits are the pink halite crystals from Seamless Lake in Trona, California.
Secondary Mineral Deposits - These form by the process of water being exposed to primary ores, by deposition from hydrothermal solutions or are formed by the crystallization of magma.
The chemical composition of the mineral dictates how it will occur within nature. Some of the common mineral classes by their chemical compositions are:
Native Minerals - Elements that occur naturally with a distinct mineral structure and no combination with another element. Some examples of elements that are known to form as native minerals are gold (Au), silver (Ag), sulfur (S), copper (Cu), graphite ((C) - loosely packed carbon) and diamonds ((C) - densely packed carbon - typically contains some impurities).
Oxides - A class of chemical compound in which an oxygen ion (O2−) pairs with an element, in many cases a positively charged metal. Some examples of this are SiO₂ - Quartz, Fe₂O₃ - Hematite, Cu₂O - Cuprite, etc.
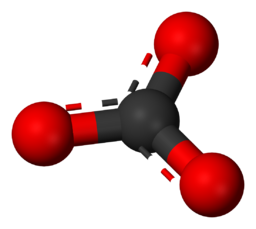
Carbonates - Minerals characterized by the presence of a carbonate ion (CO₃2-). Typically bonds to metal cations which in most cases form insoluble compounds (cannot be dissolved in water). Some examples of this are CaCO₃ - Calcite/Aragonite, FeCO₃ - Siderite, ZnCO₃ - Smithsonite, etc.
Silicates - Minerals from a family of anions that contain both silicon (Si) and oxygen (O). This salt forms a major component of the rocks throughout the lithosphere (earth’s crust). Some examples of silicates are SiO₂ - Quartz, AlKO₆Si₂ - Potassium aluminum silicate, (Fe,Mg)₂SiO₄ - Olivine, etc.
Sulfide (Sulphide) - Inorganic anion of sulfur that features the chemical formula S^2- and can involve reactions that are considered to be quite complex. The precipitation of sulfides can include reactions with heavy metals in which insoluble metal precipitates are formed. Some examples of these heavy metals that form from sulphide ions are FeS₂ - Pyrite, CuFeS₂ - Chalcopyrite, PbS - Galena, etc.
Sulfates (Sulphates) - Salts that form when sulfuric acid (H₂SO₄) reacts with another chemical. Some examples of sulfates are (Ba,Sr)SO₄ - Barite (Baryte), CaSO₄· 2H₂O - Gypsum, SrSO₄ - Celestine (Celestite), etc.
Phosphates - Minerals characterized by their presence of the complex anion (PO₄)^3−., most of which are considered quite rare in nature. Some examples of phosphates are Ca5(PO₄)(OH,F,CL) - Apatite, CuAl₆(PO₄)₄(OH)₈ · 4H₂O - Turquoise, Fe(II)3(PO4)₂· 8H2O - Vivianite, etc.
In modern times, for a mineral to be considered economically worth mining, it must exist as a concentration of useful minerals that can be worked (mined) while still obtaining a profit. The concentration must also be high enough to make extraction a worthy process. Sometimes, the mineral coming to an end within the mine can result in immediate closure of the mine. Because of this, mining can be an extra risky business as far as economics go.
Metals are currently the most important economic minerals, for they’re used for a wide variety of modern applications. These metals are extracted from metalliferous deposits that consist of the ore (sought after minerals) and in most cases the unwanted, less economic surrounding minerals known as “gangue”.
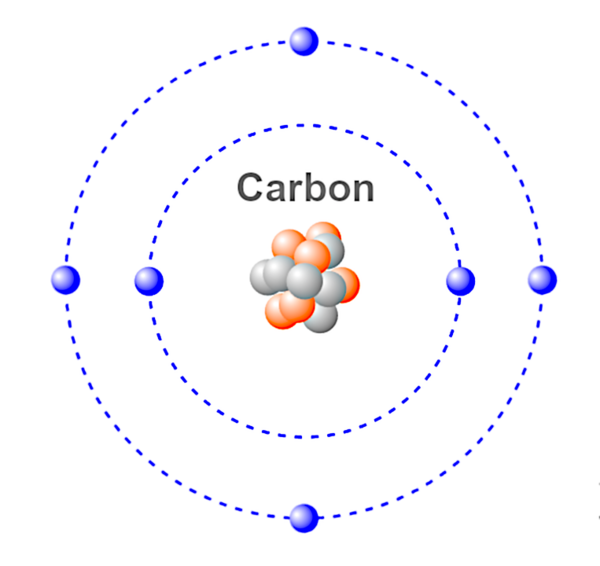
Chemical Element - A chemical element is a substance of matter whose atoms all contain the same number of protons, known as the atomic number. The periodic table is in a sense a key that was put together by Dimitri Mendeleev, a Russian chemist, to classify these atoms into elemental categories based on their number of protons. For example, a hydrogen (H) atom contains one proton, a helium (He) atom contains two protons, a lithium (Li) atom contains three protons and so on and so forth.
Chemical Compound - Made up of molecules. To be considered a chemical compound, the molecule must be composed of up to two more more different chemical elements that are bonded together.
Atom - The smallest unit of a chemical element. An atom is made up of proton(s), electron(s) and neutron(s) which together determine the charge of the atom. Atoms can lose or gain electrons, resulting in positive and negative charges known as ions.
Ion - An atom or molecule with an electrical charge that resulted from a loss or gain of one or more electrons.
Cation - A positively charged ion that is attracted to negatively charged ions. Example - Hydrogen ion H+.
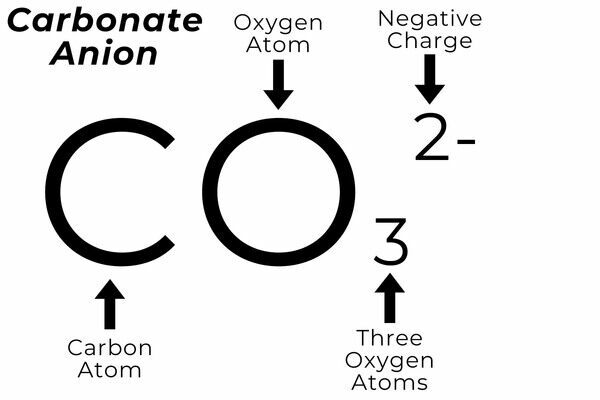
Anion - A negatively charged ion that is attracted to positively charged ions. Example - Carbonate ion is CO₃2−.
The chemical composition of a mineral is known as its elemental composition. Most minerals occur as compounds (a combination of several different elements). However, some minerals occur as chemical elements by themselves. These are known as native minerals.
What Are Crystals?
Crystals are a solid substance that possess an organized crystalline structure that’s formed from atoms. The internal arrangement of atoms often result in external plane faces like those seen on a quartz crystal, however this is not a requirement to be classified as a crystal. Crystals can be classified differently than minerals due to the fact that some materials can be considered organic crystals. Since minerals are purely inorganic, an organic crystal cannot be a mineral. For example, proteins and sugars are solids that can form crystals, however because they’re organic substances, they can’t be categorized as minerals.
Impurities within solutions that form crystals can result in color changes as well as significant crystal structure alterations. Too many impurities within a crystal lattice during crystallization can produce crystals with small to large inclusions. These inclusions can alter the shape of the crystal as well.
Minerals, Crystals or Rocks?
Most minerals will occur naturally as crystals, however not all crystals are minerals since organic crystals are not minerals at all. A mineral with the same chemical formula can form more than one type of crystal. For example, there are three polymorphs (same chemical formula, different crystalline structures) of calcium carbonate that are known as calcite, aragonite and vaterite. Calcite crystals occur in the trigonal system, aragonite crystals belong to the orthorhombic system and vaterite crystals form in the hexagonal system. These crystalline structures can vary during formation as a result of several factors which include impurities inhibiting growth patterns, the temperature of the environment during formation, the saturation of minerals within the solution, the geometry of the covalent bonds and changes in motion of the solution.
Minerals have a naturally formed, organized atomic structure with a specific chemical composition. Crystals for the most part will share these features, however the atoms are arranged in a repeating pattern that results in a crystal lattice, often presenting itself with crystal faces.
Many times you will hear people call minerals or crystals, rocks, however a “rock” is defined as a bound aggregate of minerals, mineraloids or fragments of other rocks. The word “bound” meaning the aggregation of minerals must be in a sense cemented together. For example, sand is not considered a rock even though in most cases sand grains are aggregated together. Sandstone for instance has become a rock because the sand grains have been cemented together by finer grained minerals and/or organic material, forming a relatively solid mass.
The three major classifications of rocks are igneous, metamorphic and sedimentary.
Igneous Rock - Rock that formed by the cooling and crystallization of magma within or above the lithosphere (earth’s crust). Crystals form within the magma as it begins to solidify, of which the speed of cooling can dictate the size of the crystals that form.

A close up view of porphyritic granite, a type of igneous rock. Porphyritic granite occurs when the temperature of the magma cooling changes quickly. In this instance, large crystals were allowed to form with slow cooling, only to be interrupted by a sudden decreasing temperature change that sped up the crystallization process, resulting in smaller crystals.
Sedimentary Rock - Rock that formed as a result of eroded materials from prior formed rocks being deposited along ocean floors, river beds, lake beds, etc., along with deposition of minerals from the water. Over years these depositions are compacted by natural forces, later to solidify into a solid mass (rock).
Metamorphic Rock - Rock that occurred when existing sedimentary or igneous rock was exposed to pressures and in some cases temperature changes that altered their original mineralogy.
How Do Inorganic Crystals Form?
Crystals can form from a variety of different processes, including:
Evaporite Deposits - These are mineral formations that occur as a result of processes at the earth’s surface. Crystals form from solutions containing minerals that become concentrated by dehydration/evaporation of an aqueous solution. As the fluid is slowly removed by evaporation, the concentrated minerals gather and precipitate from the water in a structured pattern that develops into a crystal. An example of precipitation deposits are the pink halite crystals from Seamless Lake in Trona, California.
Secondary Mineral Deposits - These form by the process of water being exposed to primary ores, by deposition from hydrothermal solutions or are formed by the crystallization of magma.
- Exposure to primary ores - Water that’s introduced to exposed ores (typically within a cavity) by downward percolation through rocks. This can begin chemical processes that break down the ore and redistribute the minerals along open cavity walls. Azurite and malachite are good examples of this, where aqueous solutions have moved through areas of primary copper ore and redistributed them as azurite and malachite crystals whose formation requirements include water, carbonates and copper.
- Hydrothermal fluid deposit - in most cases occurs as hydrothermal fluids make their way upward through rock, picking up minerals within the surrounding rock in the process. When an open cavity is presented to these fluids, precipitation of the minerals can occur in the form of crystals and/or a solid mass. At times the minerals within the surrounding rock can be replaced, this occurrence is known as a replacement deposit.
- Crystallization of magma - occurs as magma cools and the minerals within the magma begin to separate into groups of alike and compatible minerals. Depending on the speed of cooling, composition and atmosphere the sizes of the crystals can vary significantly. Sometimes cooling can occur too quickly, resulting in the lack of an elemental structure, obsidian (volcanic glass) being an example of this. Primarily composed of SiO₂ (quartz in most instances), obsidian has a chemical formula with the potential to be a crystal/mineral, however the lack of crystalline structure and composition variability results in its classification as a mineraloid instead.
Mineral Classes
The chemical composition of the mineral dictates how it will occur within nature. Some of the common mineral classes by their chemical compositions are:
Native Minerals - Elements that occur naturally with a distinct mineral structure and no combination with another element. Some examples of elements that are known to form as native minerals are gold (Au), silver (Ag), sulfur (S), copper (Cu), graphite ((C) - loosely packed carbon) and diamonds ((C) - densely packed carbon - typically contains some impurities).
Oxides - A class of chemical compound in which an oxygen ion (O2−) pairs with an element, in many cases a positively charged metal. Some examples of this are SiO₂ - Quartz, Fe₂O₃ - Hematite, Cu₂O - Cuprite, etc.
Carbonates - Minerals characterized by the presence of a carbonate ion (CO₃2-). Typically bonds to metal cations which in most cases form insoluble compounds (cannot be dissolved in water). Some examples of this are CaCO₃ - Calcite/Aragonite, FeCO₃ - Siderite, ZnCO₃ - Smithsonite, etc.
Silicates - Minerals from a family of anions that contain both silicon (Si) and oxygen (O). This salt forms a major component of the rocks throughout the lithosphere (earth’s crust). Some examples of silicates are SiO₂ - Quartz, AlKO₆Si₂ - Potassium aluminum silicate, (Fe,Mg)₂SiO₄ - Olivine, etc.
Sulfide (Sulphide) - Inorganic anion of sulfur that features the chemical formula S^2- and can involve reactions that are considered to be quite complex. The precipitation of sulfides can include reactions with heavy metals in which insoluble metal precipitates are formed. Some examples of these heavy metals that form from sulphide ions are FeS₂ - Pyrite, CuFeS₂ - Chalcopyrite, PbS - Galena, etc.
Sulfates (Sulphates) - Salts that form when sulfuric acid (H₂SO₄) reacts with another chemical. Some examples of sulfates are (Ba,Sr)SO₄ - Barite (Baryte), CaSO₄· 2H₂O - Gypsum, SrSO₄ - Celestine (Celestite), etc.
Phosphates - Minerals characterized by their presence of the complex anion (PO₄)^3−., most of which are considered quite rare in nature. Some examples of phosphates are Ca5(PO₄)(OH,F,CL) - Apatite, CuAl₆(PO₄)₄(OH)₈ · 4H₂O - Turquoise, Fe(II)3(PO4)₂· 8H2O - Vivianite, etc.
Economics of Crystals and Minerals
In modern times, for a mineral to be considered economically worth mining, it must exist as a concentration of useful minerals that can be worked (mined) while still obtaining a profit. The concentration must also be high enough to make extraction a worthy process. Sometimes, the mineral coming to an end within the mine can result in immediate closure of the mine. Because of this, mining can be an extra risky business as far as economics go.
Metals are currently the most important economic minerals, for they’re used for a wide variety of modern applications. These metals are extracted from metalliferous deposits that consist of the ore (sought after minerals) and in most cases the unwanted, less economic surrounding minerals known as “gangue”.
Terminology and Illustrations
Chemical Element - A chemical element is a substance of matter whose atoms all contain the same number of protons, known as the atomic number. The periodic table is in a sense a key that was put together by Dimitri Mendeleev, a Russian chemist, to classify these atoms into elemental categories based on their number of protons. For example, a hydrogen (H) atom contains one proton, a helium (He) atom contains two protons, a lithium (Li) atom contains three protons and so on and so forth.
Chemical Compound - Made up of molecules. To be considered a chemical compound, the molecule must be composed of up to two more more different chemical elements that are bonded together.
Atom - The smallest unit of a chemical element. An atom is made up of proton(s), electron(s) and neutron(s) which together determine the charge of the atom. Atoms can lose or gain electrons, resulting in positive and negative charges known as ions.
Ion - An atom or molecule with an electrical charge that resulted from a loss or gain of one or more electrons.
Cation - A positively charged ion that is attracted to negatively charged ions. Example - Hydrogen ion H+.
Anion - A negatively charged ion that is attracted to positively charged ions. Example - Carbonate ion is CO₃2−.
 Reviews
Reviews